| Development of separation & extraction techniques | |
| | The interesting field for our research is development of separation and extraction techniques, specially cloud point extraction (CPE), and its using for the extraction and preconcentration of pharmaceuticals from biological fluids or trace of elements from nutritional and environmental samples and their determination by proper spectroscopic methods.
When a micellar solution of a non–ionic surfactant is heated, it becomes turbid over a narrow temperature range, which is referred to as its cloud point temperature (CPT). | | Ahad Bavili | | | Above the CPT, such solutions separate into two isotropic phases: the “surfactant – rich (SR) phase”, which is separated from the bulk aqueous solution, and the “aqueous phase”, containing the surfactant concentration close to the CMC at that temperature. The phenomenon is reversible and upon cooling a single phase is obtained again. | |
|
The cloud point methodology is relatively simple. The steps involved in CPE prior to HPLC, GC and CE analysis have been shown in
Figure 1.
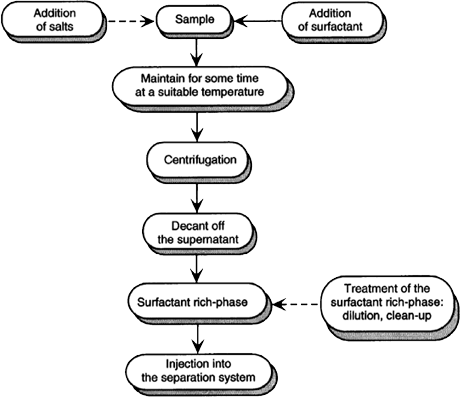
Figure 1. Steps involved in CPE prior HPLC, GC and CE analysis.
|
| Schematic representation of a conventional CPE to metal preconcentration has been shown in Figure 2.
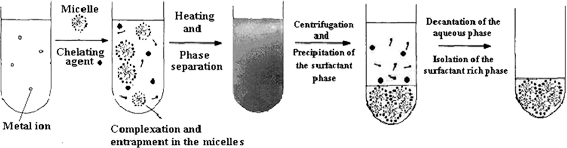 |
| Figure 2. CPE or Micelle-mediated extraction (MME) of metal ions from water samples. | |
|
Visual representation of cloud formation upon heating beyond the CPT has been shown in Figure 3.
Figure 3. Visual representation of cloud formation upon heating beyond the CPT.
The analytical potential of CPE has been demonstrated in many studies concerning the extraction and preconcentration of metal chelates, toxic organic compounds, vitamins and proteins from different real samples, and also chemical constituents from herbal products.
| 
|
A procedure for the extraction of proteins with the aqueous micellar two phase system has been shown in Figure 4.
|
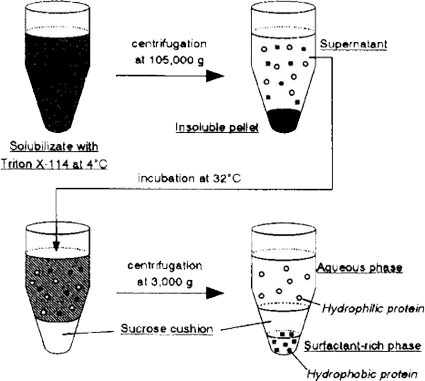 |
| Figure 4. Procedure for the CPE of brush border membrane proteins. |
|
In recent studies we have focused on development of CPE strategies to the extraction of pharmaceuticals from biological samples such as urine. Thus, the feasibility of employing non–ionic surfactant solution as an alternative and effective solvent for the extraction of pharmaceuticals from biological samples is studied.
For example, we have reported a CPE-spectrofluorimetric method for the determination of thiamine in urine. The method is based on chemical oxidation of thiamine in aqueous or urine sample to thiochrome by using ferricyanide, extraction of thiochrome to non-ionic micelles and its determination by spectrofluorimetry. This technique permits analysis and quantification of the thiamine in urine samples with simple spectrofluorimetric method instead of time-consuming and tedious HPLC method without further sample clean-up steps.
|
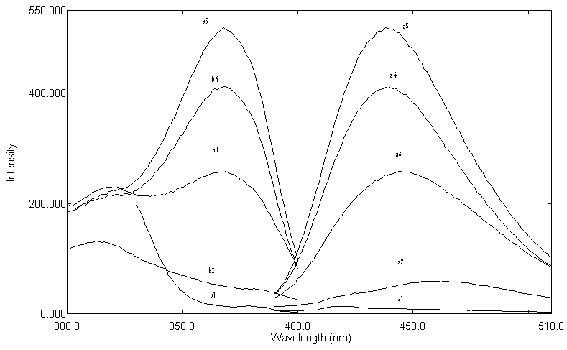
| 
|
| ....... Figure 5. Emission and excitation spectra: a1 & b1 Emission and excitation of blank; a2 & b2: Emission and excitation of urine blank; a3 & b3: Emission and excitation of thiochrome after administration of thiamine tablet; a4 & b4: Emission and excitation of thiochrome when 400 ng mL-1 thiamine spiked to the urine; a5 & b5: Emission and excitation of thiamine standard solution (500 ng mL-1) as thiochrome; 5.0×10-4 mol L-1 Fe(CN)63- ; 0.1 mol L-1 OH-, 0.1% (v/v) Triton X-114.
In other study a CPE methodology has been developed and optimized for the extraction and determination of SA, the main product of aspirin degradation, in human urine. |
| 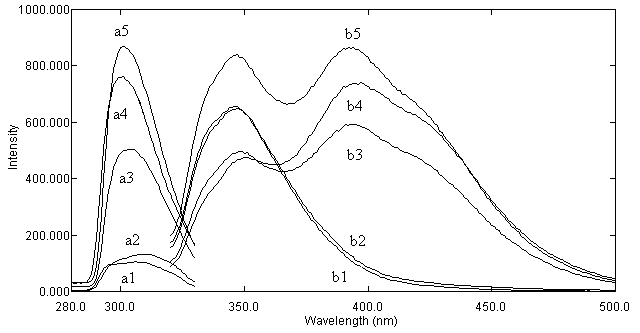
Figure 6. Excitation (a) and emission (b) spectra of: a1 and b1 – reagents. blanks; a2 and b2 – urine blank; a3 and b3 – urine spiked with SA; a4 and b4 – urine sample from healthy volunteer, who was administrated ASA (after CPE of SA from urine); a5 and b5 – 1.0 mg L.1 standard solution of SA.
|
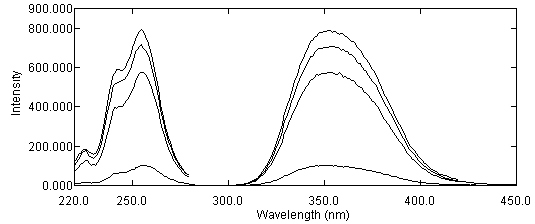
| The proposed procedure allows carrying out the analysis of SA in a simple and quick way and without long and tedious clean – up steps used in HPLC or CE. A promising approach in the area of pharmaceutical monitoring with low operation cost, simplicity of instrumentation and non – polluting respect has been presented. The proposed method can be further developed by combining of this CPE methodology with proper HPLC method for separating and detecting each of SA metabolites. Other interesting fields are: * Development of spectrofluorimetric methods for determination of pharmaceuticals in their formulations or biological samples. * Development of micellar enhanced spectrofluorimetric methods for determination of pharmaceuticals. * Indirect flame atomic absorption spectrometric determination of organic compounds. We have recently proposed simple and inexpensive spectrofluorimetric methods for determination of some drugs, such as piroxicam, propranolol, nifedipine and mefenamic acid in pharmaceutical preparations or urine. The proposed methods are based upon oxidation of these drugs with Ce(IV) and subsequent monitoring the fluorescence of the formed Ce(III). |
| 
|
|
Figure 7. Emission and excitation spectra of Ce(III): a1 & b1 Emission and excitation of Ce(III) in reagents blank; a2 & b2: Emission and excitation of Ce(III) after oxidation with 0.7 mg L-1 nifedipine (NIF) solution (prepared from NIF tablet); a3 & b3 Emission and excitation of Ce(III) after oxidation with 0.9 mg L-1 NIF solution (prepared from NIF capsule):; a4 & b4: Emission and excitation of Ce(III) after oxidation with standard solution of NIF (1.0 mg L-1); 2.5×10-5 mol L-1 Ce(IV); 1.0 mol L-1 H2SO4.
|